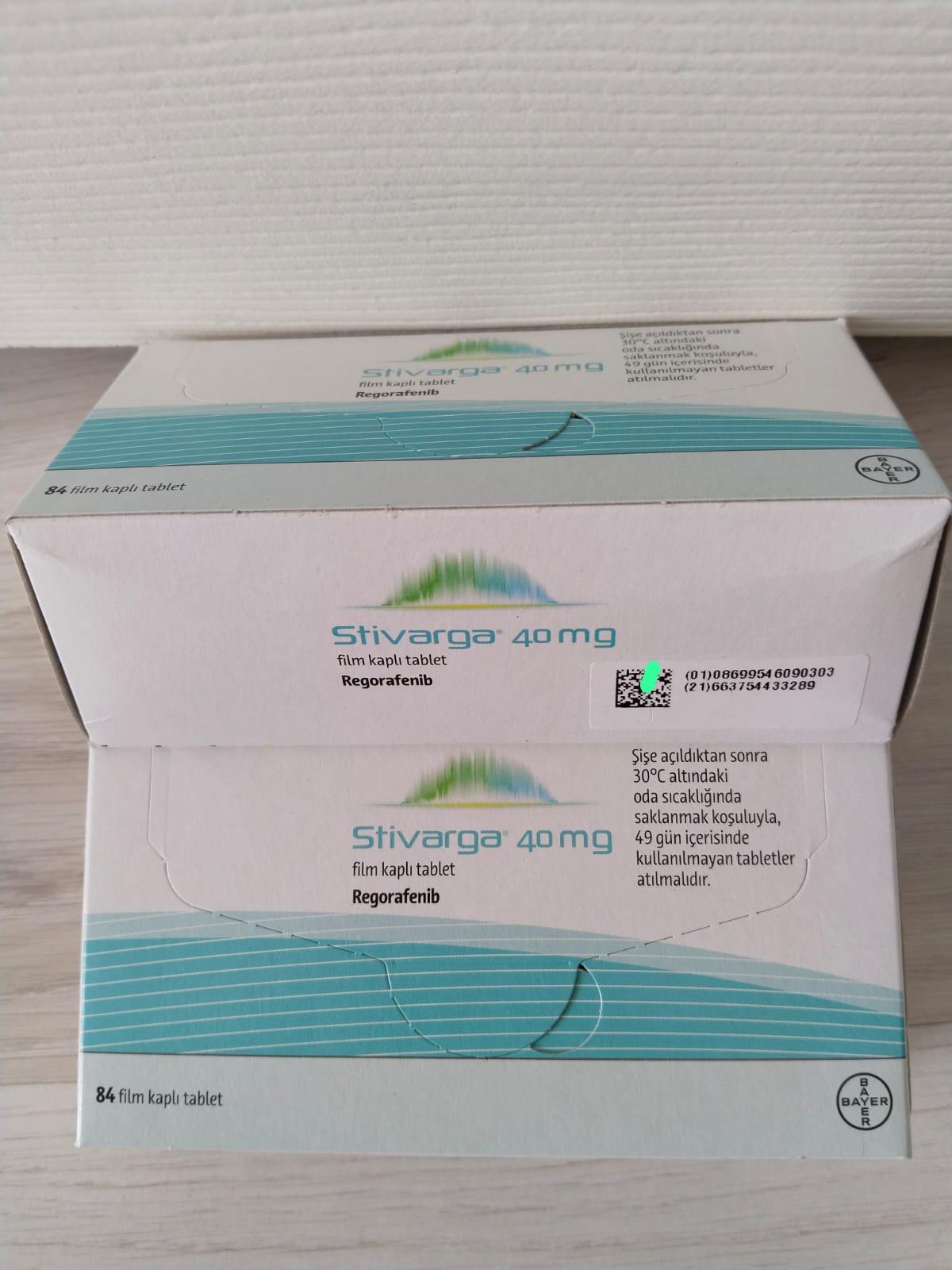
Stivarga 40 mg Film-Coated Tablets by Bayer is an advanced oral cancer treatment containing Regorafenib, a multi-kinase inhibitor. Stivarga 40 mg Regorafenib is prescribed for patients battling metastatic colorectal cancer (mCRC), gastrointestinal stromal tumors (GIST), and hepatocellular carcinoma (HCC). Known for its potent cancer-fighting properties, Stivarga 40 mg Regorafenib targets specific pathways that contribute to tumor growth and angiogenesis, helping to slow the progression of aggressive cancers.
Stivarga 40 mg Regorafenib works by inhibiting several protein kinases involved in tumor angiogenesis, oncogenesis, and the tumor microenvironment. As a targeted therapy, Stivarga 40 mg has proven efficacy in extending survival and enhancing the quality of life for patients with advanced and hard-to-treat cancers.
🔥 Key Benefits & Uses of Stivarga 40 mg Regorafenib:
- Metastatic Colorectal Cancer (mCRC): Stivarga 40 mg is used when standard chemotherapy treatments have failed, offering a second-line treatment option.
- Gastrointestinal Stromal Tumors (GIST): Stivarga 40 mg Regorafenib is effective for patients who no longer respond to imatinib or sunitinib.
- Hepatocellular Carcinoma (HCC): Stivarga 40 mg is prescribed after the progression of liver cancer following sorafenib treatment.
- Anti-Tumor Activity: Stivarga 40 mg Regorafenib blocks multiple pathways involved in tumor growth and blood vessel formation.
- Oral Treatment: Stivarga 40 mg Film-Coated Tablets offer a convenient oral option for cancer patients.
💊 How to Use Stivarga 40 mg Regorafenib:
- Dosage: The standard dose is 160 mg (4 tablets of Stivarga 40 mg) once daily for 21 days, followed by a 7-day rest period.
- Administration: Stivarga 40 mg should be taken with a low-fat meal at the same time each day.
- Duration: Treatment cycles are determined by the healthcare provider based on the patient’s response and tolerance.
- Missed Dose: If a dose is missed, it should not be doubled; continue with the next scheduled dose.
🧊 Storage Instructions:
- Store Stivarga 40 mg Tablets below 30°C.
- Once the bottle is opened, use Stivarga 40 mg within 49 days.
- Keep Stivarga 40 mg Regorafenib in its original bottle to protect from moisture.
- Keep out of reach of children.
⚠️ Potential Side Effects of Stivarga 40 mg Regorafenib:
- Fatigue and weakness.
- Diarrhea and nausea.
- Hand-foot skin reactions.
- Hypertension (high blood pressure).
- Liver function abnormalities.
Patients should seek medical advice if severe side effects occur during Stivarga 40 mg treatment.
🌍 Why Choose Stivarga 40 mg Regorafenib by Bayer?
- Manufactured by Bayer, a globally recognized pharmaceutical company.
- Effective multi-kinase inhibitor targeting multiple cancer pathways.
- Extends survival and delays disease progression in mCRC, GIST, and HCC.
- Recommended by oncologists worldwide for resistant cancers.
- Convenient oral tablet form allows at-home treatment.
✅ Real Patient Reviews:
“After exhausting other treatments, Stivarga 40 mg Regorafenib became my last hope. It slowed the progression of my colorectal cancer and gave me precious extra time with my loved ones.” – Patient Testimonial
🔗 Official Resource:
Bayer Oncology Official Page – For comprehensive medical information and prescribing guidelines.

Değerlendirmeler
Henüz değerlendirme yapılmadı.